
Chemistry, 21.03.2020 11:11 alisonnn101
Calculate ΔS for the isothermal compression of 3.05 mol of Cu(s) from 1.00 bar to 1370. bar at 298 K. α=0.492×10−4K−1,κT=0.78×10−6 bar−1, and the density is 8.92 g⋅cm−3.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Calculate ΔS for the isothermal compression of 3.05 mol of Cu(s) from 1.00 bar to 1370. bar at 298 K...
Questions in other subjects:

Mathematics, 23.03.2021 01:40

Mathematics, 23.03.2021 01:40

Mathematics, 23.03.2021 01:40


History, 23.03.2021 01:40

Health, 23.03.2021 01:40



Mathematics, 23.03.2021 01:40

 ) is as follows.
) is as follows.
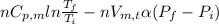


 = -0.146 J/K
= -0.146 J/K

