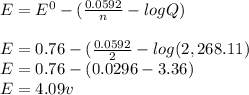Refer to the following standard reduction half-cell potentials at 25∘C:
VO2+(aq)+Ni2+(aq)2H+(aq)++2e−e−→ →Ni(s)VO2+(aq) +H2O(l)E∘=−0.23V E∘=0.99V
An electrochemical cell is based on these two half-reactions: Oxidation:Reduction:
Ni(s)VO2+(aq,0.083M)+2H+(aq,1.1M)+e −→→Ni2+(aq,2.5M)+2e−VO2+(aq,2.5M)+H 2O(l)
Calculate the cell potential under these nonstandard concentrations.
Express the cell potential to two decimal places and include the appropriate units.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1
You know the right answer?
Refer to the following standard reduction half-cell potentials at 25∘C:
VO2+(aq)+Ni2+(a...
VO2+(aq)+Ni2+(a...
Questions in other subjects:






Social Studies, 17.04.2020 04:35

Mathematics, 17.04.2020 04:35



![E = E^{0} - [(\frac{0.0592}{n} · log Q)]](/tpl/images/0557/5710/84c6b.png)
 = Cell potential (standard conditions)
= Cell potential (standard conditions)
 + e- -->
+ e- -->  -0.23
-0.23 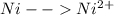 + 2 e- +0.99
+ 2 e- +0.99 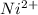 + 2e- 0.76 v
+ 2e- 0.76 v ![Q = \frac{[(VO_{2}+]^{2}* [Ni^{2+} }{[VO_{2+}]^{2} * Ni }]](/tpl/images/0557/5710/56e6b.png)
![[VO_{2} ^{2+}] = 2.5 M](/tpl/images/0557/5710/bdce1.png)
![[VO_{2}+] = 0.083 M](/tpl/images/0557/5710/9505a.png)
![[Ni^{2+}] = 2.5 M](/tpl/images/0557/5710/3a699.png)
![[Ni] = 1 M, it is a pure solid, so its activity in Q is unit (1). It is also applied for pure liquids.](/tpl/images/0557/5710/4494d.png)