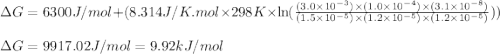Given the following: [G3P] = 1.5x10-5M; [BPG] = 3.0x10-3M ; [NAD+] = 1.2x10-5M; [NADH]=1.0x10-4 ; [HPO42-]= 1.2x10-5 M; pH = 7.5 ; DGo=6.3 kJ/mol
Glyceraldehyde3-phosphate + NAD+ + HPO42- ---> 1,3-Biphosphoglycerate + NADH + H+
Predict whether this reaction will be spontaneous.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3


Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Given the following: [G3P] = 1.5x10-5M; [BPG] = 3.0x10-3M ; [NAD+] = 1.2x10-5M; [NADH]=1.0x10-4 ; [H...
Questions in other subjects:










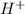 concentration, we use the equation:
concentration, we use the equation:![pH=-\log[H^+]](/tpl/images/0557/5671/cf945.png)
![7.5=-\log [H^+]](/tpl/images/0557/5671/95063.png)
![[H^+]=10^{-7.5)=3.1\times 10^{-8}M](/tpl/images/0557/5671/67b53.png)

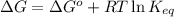
 = free energy of the reaction
= free energy of the reaction = standard Gibbs free energy = 6.3 kJ/mol = 6300 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 6.3 kJ/mol = 6300 J/mol (Conversion factor: 1kJ = 1000J)![25^oC=[273+25]K=298K](/tpl/images/0557/5671/0e82f.png)
 = Ratio of concentration of products and reactants =
= Ratio of concentration of products and reactants = ![\frac{[BPG][NaDH][H^+]}{[G_3P][NAD^+][HPO_4^{2-}]}](/tpl/images/0557/5671/dd6ba.png)
![[BPG]=3.0\times 10^{-3}M](/tpl/images/0557/5671/38e24.png)
![[NADH]=1.0\times 10^{-4}M](/tpl/images/0557/5671/94c0b.png)
![[H^+]=3.1\times 10^{-8}M](/tpl/images/0557/5671/2f9b6.png)
![[G_3P]=1.5\times 10^{-5}M](/tpl/images/0557/5671/aafff.png)
![[NAD^+]=1.2\times 10^{-5}M](/tpl/images/0557/5671/711eb.png)
![[HPO_4^{2-}]=1.2\times 10^{-5}M](/tpl/images/0557/5671/2ab39.png)