Chemistry, 21.03.2020 08:35 alexusjones6042
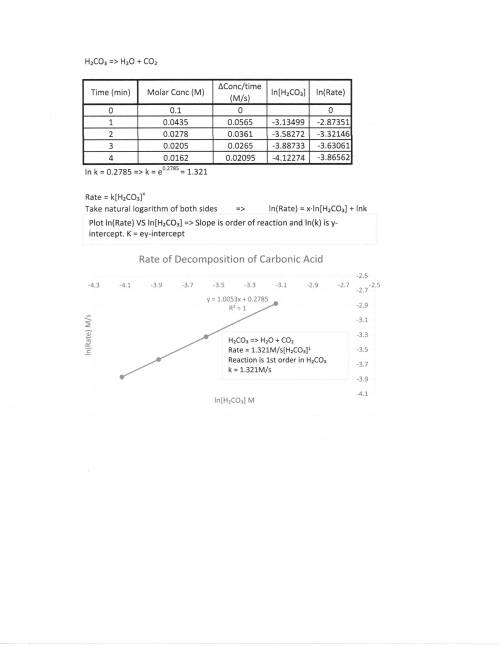
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
He fills a reaction vessel with H2CO3 and measures its concentration as the reaction proceeds:
time (minutes) H2CO3
0 0.100M
1.0 0.0435M
2.0 0.0278M
3.0 0.0205M
4.0 0.0162M
write the reate law for this reaction: k ?
calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbols.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
Questions in other subjects:



Social Studies, 30.09.2019 14:00

Mathematics, 30.09.2019 14:00

Mathematics, 30.09.2019 14:00

History, 30.09.2019 14:00




History, 30.09.2019 14:00