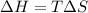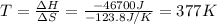Chemistry, 21.03.2020 05:35 milkshakegrande101
For a particular reaction, Δ H ∘ = − 46.7 kJ and Δ S ∘ = − 123.8 J/K. Assuming these values change very little with temperature, at what temperature does the reaction change from nonspontaneous to spontaneous?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
For a particular reaction, Δ H ∘ = − 46.7 kJ and Δ S ∘ = − 123.8 J/K. Assuming these values change v...
Questions in other subjects:



Arts, 06.01.2021 05:30




Health, 06.01.2021 05:30

Chemistry, 06.01.2021 05:30

Arts, 06.01.2021 05:30

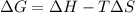
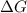 = Gibbs free energy
= Gibbs free energy 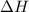 = enthalpy change = -46.7 kJ= -46700 J
= enthalpy change = -46.7 kJ= -46700 J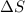 = entropy change = -123.8 J/K
= entropy change = -123.8 J/K