
Chemistry, 21.03.2020 04:52 jackieanguiano3700
He following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlorine. Cl2 ⇌ 2Cl (fast, reversible) Cl + CHCl3 → HCl + CCl3 (slow) Cl + CCl3 → CCl4 (fast) What rate law does this mechanism predict? (Choose from the list below and enter your answers in alphabetical order, e. g. ABC ). A)k G) [CCl3]1/2 M) [HCl]2 B) [Cl] H) [HCl]1/2 N) [Cl2]2 C) [CHCl3] I) [Cl2]1/2 O) [Cl]2 D) [CCl3] J) [Cl]1/2 P) [CHCl3]2 E) [HCl] K) [CHCl3]1/2 F) [Cl2] L) [CCl3]2 Tries 0/99 Decide which of the following reactive intermediates could exist for the reaction above. Cl Cl2 CHCl3 CCl3 CCl4 Tries 0/99

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
He following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlori...
Questions in other subjects:


Mathematics, 12.07.2019 16:30



Mathematics, 12.07.2019 16:30


History, 12.07.2019 16:30


Mathematics, 12.07.2019 16:30

Mathematics, 12.07.2019 16:30

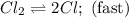
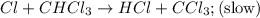
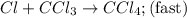
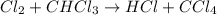
![\text{Rate}=K_2[Cl][CHCl_3]](/tpl/images/0557/3211/4079f.png) ......(1)
......(1)![K_1=\frac{[Cl]^2}{[Cl_2]}](/tpl/images/0557/3211/5c3f3.png)
![[Cl]=\sqrt{K_1[Cl_2]}](/tpl/images/0557/3211/af496.png)
![K_3=\frac{[CCl_4]}{[CHCl_3][Cl]}](/tpl/images/0557/3211/3404e.png)
![[Cl]=\frac{[CCl_4]}{K_3\times [CCl_3]}](/tpl/images/0557/3211/7b25a.png)
![\text{Rate}=K_2(\sqrt{K_1[Cl_2]}\times (\frac{[CCl_4]}{K_3[CCl_3]})[CHCl_3]\\\\\text{Rate}=k[Cl]^{1/2}[CCl_4][CCl_3]^{-1}[CHCl_3]](/tpl/images/0557/3211/b7240.png)


