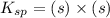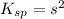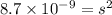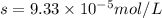Chemistry, 21.03.2020 03:31 samanthasheets8925
Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Calculate the solubility of ( = ) in moles per liter. Ignore any acid–base properties. s = mol/L Cal...
Questions in other subjects:


History, 02.10.2019 04:00

History, 02.10.2019 04:00


Mathematics, 02.10.2019 04:00


Biology, 02.10.2019 04:00


Mathematics, 02.10.2019 04:00

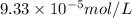
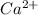 ion and
ion and 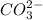 ion.
ion.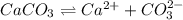
![K_{sp}=[Ca^{2+}][CO_3^{2-}]](/tpl/images/0557/2415/6c60d.png)