Chemistry, 21.03.2020 03:15 gshreya2005
2.00 liters of hydrogen, originally at 25.0 °C and 750.0 mm of mercury, are heated until a volume of 20.0 liters and a pressure of 3.50 atmospheres is reached. What is the new temperature?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 19:30, jessixa897192
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

You know the right answer?
2.00 liters of hydrogen, originally at 25.0 °C and 750.0 mm of mercury, are heated until a volume of...
Questions in other subjects:


History, 25.03.2020 21:37


Mathematics, 25.03.2020 21:37


Mathematics, 25.03.2020 21:37



History, 25.03.2020 21:37

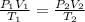
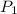 = initial pressure of gas = 750.0 mm Hg = 0.98 atm (760mmHg=1atm)
= initial pressure of gas = 750.0 mm Hg = 0.98 atm (760mmHg=1atm)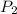 = final pressure of gas = 3.50 atm
= final pressure of gas = 3.50 atm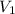 = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L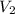 = final volume of gas = 20.0 L
= final volume of gas = 20.0 L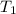 = initial temperature of gas =
= initial temperature of gas = 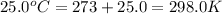
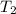 = final temperature of gas = ?
= final temperature of gas = ?