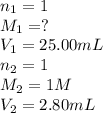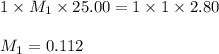Chemistry, 21.03.2020 03:13 ellisc7044
1. Determine the volume of 1M NaOH that is required to reach the equivalence point with 25.00 mL of HCl (of unknown concentration). From there, calculate the original concentration of the unknown HCl solution.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
1. Determine the volume of 1M NaOH that is required to reach the equivalence point with 25.00 mL of...
Questions in other subjects:




Social Studies, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00


Physics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


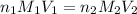
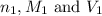 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 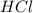
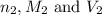 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.