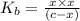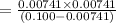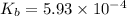Chemistry, 21.03.2020 03:01 bettybales1986
Aspirin (acetylsalicylic acid, C9H8O4) is a weak monoprotic acid. To determine its acid-dissociation constant, a student dissolved 2.00 g of aspirin in 0.600 L of water and measured the pH.
What was the Ka value calculated by the student if thepH of the solution was 2.62?
A 0.100 M solution of ethylamine (C2H5NH2) has a pH of 11.87.
Calculate the Kb for ethylamine.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 21.06.2019 20:10, maribel2421
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

You know the right answer?
Aspirin (acetylsalicylic acid, C9H8O4) is a weak monoprotic acid. To determine its acid-dissociation...
Questions in other subjects:


Mathematics, 25.03.2021 05:40

Physics, 25.03.2021 05:40







Mathematics, 25.03.2021 05:40

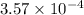 was the
was the  value calculated by the student.
value calculated by the student.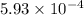 was the
was the 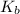 of ethylamine value calculated by the student.
of ethylamine value calculated by the student. value of Aspirin solution = 2.62
value of Aspirin solution = 2.62![pH=-\log[H^+]](/tpl/images/0557/1305/cf945.png)
![[H^+]=10^{-2.62}=0.00240 M](/tpl/images/0557/1305/aef02.png)
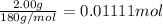
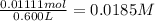
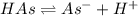
![K_a=\frac{[As^-][H^+]}{[HAs]}](/tpl/images/0557/1305/c8f9f.png) :
: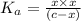

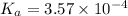
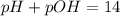

![pOH=-\log[OH^-]](/tpl/images/0557/1305/fe336.png)
![[OH^-]=10^{-2.13}=0.00741 M](/tpl/images/0557/1305/fa327.png)
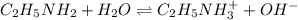
![K_b=\frac{[C_2H_5NH_3^{+}][OH^-]}{[C_2H_5NH_2]}](/tpl/images/0557/1305/63c9d.png) :
: