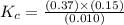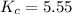Chemistry, 21.03.2020 02:58 alexisfaithsmith
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus trichloride, the following concentrations are obtained: 0.010 mol/L PCl5, 0.15 mol/l PCl3 and 0.37 mol/L Cl2. Determine the Keq for the reaction

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:00, sCoTtYbOy5329
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1

Chemistry, 23.06.2019 06:00, mirzakasumovic8926
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

Chemistry, 23.06.2019 08:00, ira51
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus...
Questions in other subjects:

Mathematics, 02.02.2021 23:30


Mathematics, 02.02.2021 23:30





Mathematics, 02.02.2021 23:30


Physics, 02.02.2021 23:30

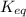 for the reaction is 5.55
for the reaction is 5.55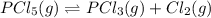
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/0557/1142/ffe89.png)