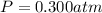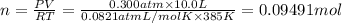Chemistry, 21.03.2020 02:59 carleygalloway103
0.100 mol of CaCO3 and 0.100 mol CaO are placed in an 10.0 L evacuated container and heated to 385 K. When equilibrium is reached the pressure of CO2 is 0.220 atm. 0.300 atm of CO2 is added, while keeping the temperature constant and the system is allowed to reach again equilibrium. What will be the final mass of CaCO3

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
0.100 mol of CaCO3 and 0.100 mol CaO are placed in an 10.0 L evacuated container and heated to 385 K...
Questions in other subjects:


Social Studies, 15.02.2022 04:20

Physics, 15.02.2022 04:20




Social Studies, 15.02.2022 04:20

Mathematics, 15.02.2022 04:20

Mathematics, 15.02.2022 04:20

Business, 15.02.2022 04:20

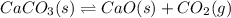
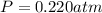
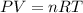 ( ideal gas equation)
( ideal gas equation)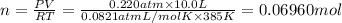
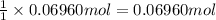 calcium carbonate
calcium carbonate