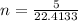Chemistry, 21.03.2020 02:57 sherlock19
Gas mixture consists of N2, O2, and Ne, where the mole fraction of N2 is 0.55 and the mole fraction of Ne is 0.25. If the mixture is at STP in a 5.0 L container, how many molecules of O2 are present?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Gas mixture consists of N2, O2, and Ne, where the mole fraction of N2 is 0.55 and the mole fraction...
Questions in other subjects:





History, 09.06.2020 04:57

Mathematics, 09.06.2020 04:57



Mathematics, 09.06.2020 04:57

Mathematics, 09.06.2020 04:57