
Chemistry, 21.03.2020 02:02 daartist3121
2 H2+O2=2 H2O
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H2O was produced?
Show your work.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1



Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
2 H2+O2=2 H2O
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H...
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H...
Questions in other subjects:


English, 11.08.2021 19:50

Mathematics, 11.08.2021 19:50

Social Studies, 11.08.2021 19:50


Mathematics, 11.08.2021 19:50




Health, 11.08.2021 19:50

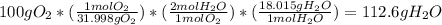
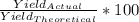 =
= 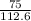 = 66.60403646
= 66.60403646


