
Chemistry, 20.03.2020 22:41 sarahsompayrac
Sarah was studying phase change in science class. She believed that cold water would begin boiling sooner than warmer water. Sarah and his father did the following investigation. Hypothesis: The lower the water’s starting temperature, the quicker the water will start to boil. Experimental Design: Sarah and her dad used three different water temperatures. They measured 50 ml of water into 100 ml beakers. The temperature of the water was recorded. They placed the beakers in a large pan of water and placed the water on the burner of the stove. Sarah timed how long it took each container to boil. They also ran the experiment three times and took an average of their data. You can see the data in the table below.
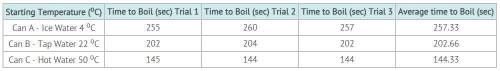
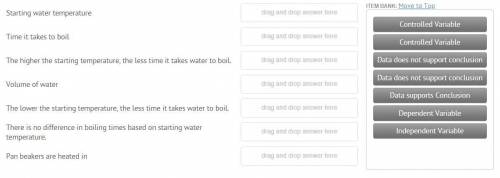

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Sarah was studying phase change in science class. She believed that cold water would begin boiling s...
Questions in other subjects:

Business, 03.05.2020 12:58




English, 03.05.2020 12:58


Arts, 03.05.2020 12:58

Biology, 03.05.2020 12:58





