
Chemistry, 20.03.2020 12:25 cupcake20019peehui
2. Hydrogen fluoride can be produced from elemental fluorine and hydrogen according to the reaction H2(g) + F2(g) 2HF(g). The reaction has an equilibrium constant, Kc, of 7.75 x 10 2 at a certain temperature. a. Calculate the equilibrium concentration of HF(g) if 5.750 mol of H2 and F2 are introduced into a 1.500 L flask.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 07:00, jpaintballer1
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1
You know the right answer?
2. Hydrogen fluoride can be produced from elemental fluorine and hydrogen according to the reaction...
Questions in other subjects:


Mathematics, 23.11.2020 19:10


Chemistry, 23.11.2020 19:10

Mathematics, 23.11.2020 19:10


Physics, 23.11.2020 19:10


Computers and Technology, 23.11.2020 19:10

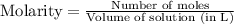
![[H_2]=\frac{5.750 mol}{1.500 L}=3.833 M](/tpl/images/0556/1625/21097.png)
![[F_2]=\frac{5.750 mol}{1.500 L}=3.833 M](/tpl/images/0556/1625/31341.png)
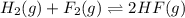
![K_c=\frac{[HF]^2}{[H_2][F_2]}](/tpl/images/0556/1625/a2854.png)
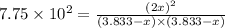
![[HF]=2x=2\times 3.576 M=7.152 M\approx 7.15 M](/tpl/images/0556/1625/f33c9.png)


