
Chemistry, 20.03.2020 12:03 cedricevans41p4j3kx
A 140.0-g sample of water at 25.0°C is mixed with 108.6 g of a certain metal at 100.0°C. After thermal equilibrium is established, the (final) temperature of the mixture is 29.6°C. What is the specific heat capacity of the metal, assuming it is constant over the temperature range concerned?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
A 140.0-g sample of water at 25.0°C is mixed with 108.6 g of a certain metal at 100.0°C. After therm...
Questions in other subjects:










Mathematics, 26.02.2020 06:02

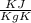
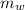 = 140 gm = 0.14 kg
= 140 gm = 0.14 kg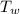 = 25°c = 298 K
= 25°c = 298 K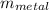 = 108.6 gm = 0.1086 kg
= 108.6 gm = 0.1086 kg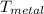 = 100°c = 373 K
= 100°c = 373 K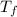 = 29.6°c = 302.6 K
= 29.6°c = 302.6 K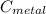 × (
× ( 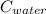 × (
× ( 

