Chemistry, 20.03.2020 12:02 PAADUUUgma
Consider the first-order reaction described by the equation At a certain temperature, the rate constant for this reaction is 5.82 × 10 − 4 s − 1 5.82×10−4 s−1 . Calculate the half-life of cyclopropane at this temperature.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mimithurmond03
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

You know the right answer?
Consider the first-order reaction described by the equation At a certain temperature, the rate const...
Questions in other subjects:


Mathematics, 20.09.2020 01:01



Mathematics, 20.09.2020 01:01

Chemistry, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01

Chemistry, 20.09.2020 01:01

History, 20.09.2020 01:01

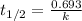
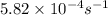
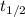 = half life of the reaction = ?
= half life of the reaction = ?