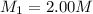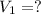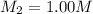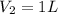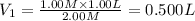A student wants to prepare 1.00 L of a 1.00 M solution of NaOH (molar mass 40.00 g/mol). If solid NaOH is available, how would the student prepare this solution? If 2.00 MNaOH is avail- able, how would the student prepare the solution? To help insure three significant figures in the NaOH molarity, to how many sig- nificant figures should the volumes and mass be determined?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
A student wants to prepare 1.00 L of a 1.00 M solution of NaOH (molar mass 40.00 g/mol). If solid Na...
Questions in other subjects:


History, 29.08.2019 00:30


Mathematics, 29.08.2019 00:30

Advanced Placement (AP), 29.08.2019 00:30


Social Studies, 29.08.2019 00:30



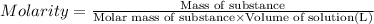
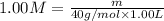
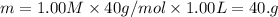
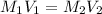 (dilution equation)
(dilution equation)