
Chemistry, 20.03.2020 11:59 martinezzz2294
1.00 mole of an ideal monoatomic gas at STP first undergoes isothermal expansion so that the volume at b is 2.5 times the volume at a. Next, heat is extracted at a constant volume so that the pressure drops. The gas is then compressed adiabatically back to the original state. Calculate the pressure at c.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
1.00 mole of an ideal monoatomic gas at STP first undergoes isothermal expansion so that the volume...
Questions in other subjects:


Mathematics, 25.08.2019 11:10




Mathematics, 25.08.2019 11:10




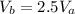

![\frac{P_c}{P_a}=[\frac{V_a}{V_c}]^{(2/3)](/tpl/images/0556/1040/0de96.png)
![\frac{P_c}{1.0 atm}=[\frac{1}{2.5}]^{(2/3)](/tpl/images/0556/1040/c3aec.png)



