
Chemistry, 20.03.2020 10:26 cearadenney7067
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy Ea= 50.0 KJ/mol. If the rate constant of this reaction is 0.14M-1 s-1 at -8.0what will the rate constant be at 56.0 ?
Round your answer to significant digits
k= M-1s-1

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an acti...
Questions in other subjects:

Mathematics, 22.08.2020 20:01




Mathematics, 22.08.2020 20:01




English, 22.08.2020 20:01

Computers and Technology, 22.08.2020 20:01

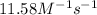
![\ln(\frac{K_{56^oC}}{K_{-8^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0555/9521/c3b12.png)
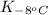 = equilibrium constant at -8°C =
= equilibrium constant at -8°C = 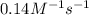
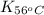 = equilibrium constant at 56°C = ?
= equilibrium constant at 56°C = ?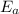 = Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)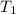 = initial temperature =
= initial temperature = ![-8^oC=[273-8]K=265K](/tpl/images/0555/9521/87944.png)
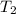 = final temperature =
= final temperature = ![56^oC=[273+56]K=329K](/tpl/images/0555/9521/333d6.png)
![\ln(\frac{K_{56^oC}}{0.14})=\frac{50000J}{8.314J/mol.K}[\frac{1}{265}-\frac{1}{329}]\\\\K_{56^oC}=11.58M^{-1}s^{-1}](/tpl/images/0555/9521/de71d.png)


