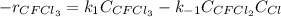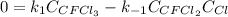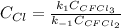Chemistry, 20.03.2020 10:54 diwashkandel6pe02af
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the rate constant of this reaction, and k1 stand for the rate constant of the reverse reaction Write an expression that gives the equilibrium concentration of Cl in terms of k, k_1, and the equilibrium concentrations of CFCI3 and CFCI2 1. K-1 [ci]

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, christopherluckey7
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the r...
Questions in other subjects:


Mathematics, 05.10.2020 21:01





History, 05.10.2020 21:01



Chemistry, 05.10.2020 21:01

![[Cl]_{eq}=\frac{k_1[CFCl_3]_{eq}}{k_{-1}[CFCl_2]_{eq}}](/tpl/images/0555/9861/640b9.png)