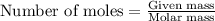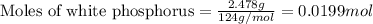Chemistry, 20.03.2020 10:03 xXCoryxKenshinXx
2.478 g of white phosphorus was used to make phosphine according to the equation: P₄(s) + 3OH⁻(aq) + 3H₂O(l) → PH₃(g) + 3H₂PO₂⁻(aq) Calculate the amount, in mol, of white phosphorus

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2



Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
2.478 g of white phosphorus was used to make phosphine according to the equation: P₄(s) + 3OH⁻(aq) +...
Questions in other subjects:

Chemistry, 20.01.2020 02:31


Geography, 20.01.2020 02:31




Mathematics, 20.01.2020 02:31

Business, 20.01.2020 02:31