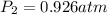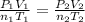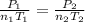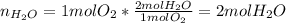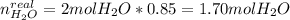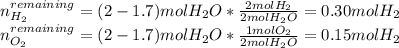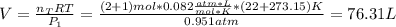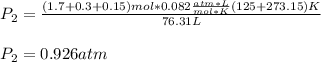A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by the reaction 2 H2 (g) + O2 (g) → 2 H2O (g) The total pressure in the container is 0.951 atm at 22°C before the reaction. What is the final pressure in the container after the reaction, with a final temperature of 125°C, no volume change, and an 85.0% yield?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1



Chemistry, 23.06.2019 10:30, sbelgirl2000
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by...
Questions in other subjects:

History, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

Computers and Technology, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01


History, 13.07.2020 20:01



Computers and Technology, 13.07.2020 20:01

Biology, 13.07.2020 20:01