Chemistry, 20.03.2020 10:04 morkitus13
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequency of 8.225 × 1016 Hz. Identify the ion. (Enter the symbol of the element in the first box, and its charge in the second.)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

You know the right answer?
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequ...
Questions in other subjects:

Geography, 25.10.2019 16:43




History, 25.10.2019 16:43


Social Studies, 25.10.2019 16:43



Social Studies, 25.10.2019 16:43

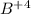
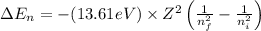
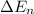 = change in energy
= change in energy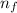 = Higher energy level =
= Higher energy level = 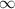
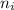 = Lower energy level = 1
= Lower energy level = 1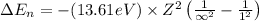
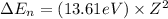 ............(1)
............(1)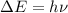
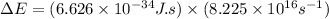
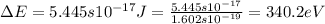 .......(2)
.......(2)