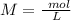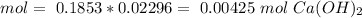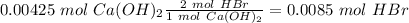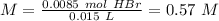Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2


Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
The titration of 15.00 mL of hydrobromic acid required 22.96 mL of a 0.1853 M calcium hydroxide to r...
Questions in other subjects:

Mathematics, 22.07.2021 19:10

Mathematics, 22.07.2021 19:10

Mathematics, 22.07.2021 19:10

Mathematics, 22.07.2021 19:10



Mathematics, 22.07.2021 19:20

Mathematics, 22.07.2021 19:20


Mathematics, 22.07.2021 19:20