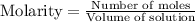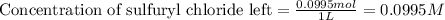Chemistry, 20.03.2020 09:55 solobiancaa
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g) follows first-order kinetics. At 320◦C the rate constant is 2.2 × 10−5 sec−1 . If one started with a sample containing 0.16 moles of sulfuryl chloride per liter at 320◦C, what concentration would be left after 6.00 hours?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g)...
Questions in other subjects:

Mathematics, 19.07.2019 10:50





English, 19.07.2019 10:50

Computers and Technology, 19.07.2019 10:50



Mathematics, 19.07.2019 10:50

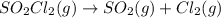
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0555/8016/f1041.png)
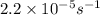
![[A_o]](/tpl/images/0555/8016/dc622.png) = initial amount of the sample = 0.16 moles
= initial amount of the sample = 0.16 moles![2.2\times 10^{-5}=\frac{2.303}{21600}\log\frac{0.16}{[A]}](/tpl/images/0555/8016/ba4a8.png)
![[A]=0.0995moles](/tpl/images/0555/8016/152d1.png)