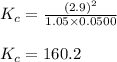Chemistry, 20.03.2020 09:07 khalilh1206
The following reaction was performed in a sealed vessel at 772 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.90M and [I2]=2.95M . The equilibrium concentration of I2 is 0.0500 M . What is the equilibrium constant, Kc, for the reaction at this temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, MansellS5529
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1


Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
The following reaction was performed in a sealed vessel at 772 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, on...
Questions in other subjects:


Mathematics, 25.09.2021 01:20

English, 25.09.2021 01:20





History, 25.09.2021 01:20

Mathematics, 25.09.2021 01:20

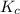 for the given equation is 160.2
for the given equation is 160.2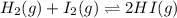
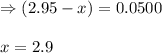
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0555/7227/62646.png)