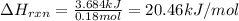Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, yellowmiki6647
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
2. When 15.3 g of sodium nitrate, NaNO3,was dissolved in water in a calorimeter, the temperature fel...
Questions in other subjects:



Arts, 20.01.2021 19:40

History, 20.01.2021 19:40






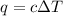
 = change in temperature =
= change in temperature = 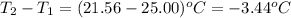
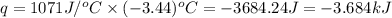
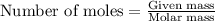
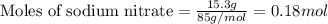
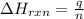
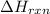 = enthalpy change of the reaction
= enthalpy change of the reaction