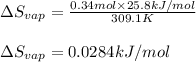Chemistry, 20.03.2020 07:24 ramanpreet
The molar heat of vaporization of pentane is 25.8 kJ·mol−1, and the boiling point of pentane is 36.1°C. Calculate the value of ΔvapS for the vaporization of 0.34 mole of pentane.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
The molar heat of vaporization of pentane is 25.8 kJ·mol−1, and the boiling point of pentane is 36.1...
Questions in other subjects:





Mathematics, 28.09.2020 07:01

Mathematics, 28.09.2020 07:01




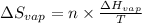
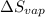 = Entropy change of vaporization = ?
= Entropy change of vaporization = ?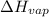 = molar heat of vaporization = 25.8 kJ/mol
= molar heat of vaporization = 25.8 kJ/mol![36.1^oC=[36.1+273]K=309.1K](/tpl/images/0555/6193/0c07c.png)