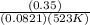A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 would be 0.50 atm at 523 K. However, PCl5 decomposes to gaseous PCl3 and Cl2, and the actual pressure in the flask was found to be 0.78 atm. Calculate Kp for the decomposition reaction PCl5(g) PCl3(g) Cl2(g) at 523 K. Also, calculate K at this temperature.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 wo...
Questions in other subjects:


English, 22.08.2020 20:01

English, 22.08.2020 20:01


Engineering, 22.08.2020 20:01





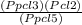 Kp =
Kp = 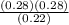 Kp = 0.35
Kp = 0.35