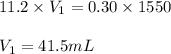Chemistry, 20.03.2020 05:27 harleyy6802
A concentration solution of H2so4 is 59.4% by mass (m/m) and has a density of 1.83 g/mL. How many mL of the solution would be required to prepare 1550 mL of a .30M solution of the acid

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
A concentration solution of H2so4 is 59.4% by mass (m/m) and has a density of 1.83 g/mL. How many mL...
Questions in other subjects:




History, 30.08.2019 23:10



Mathematics, 30.08.2019 23:10


Mathematics, 30.08.2019 23:10

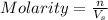
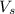 = volume of solution in L
= volume of solution in L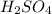 in 100 g of solution
in 100 g of solution 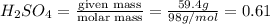
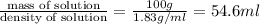
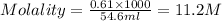
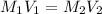
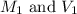 are the molarity and volume of stock acid which is
are the molarity and volume of stock acid which is 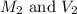 are the molarity and volume of dilute acid which is
are the molarity and volume of dilute acid which is