

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

You know the right answer?
A student dissolves 0.0100 mole of an unknown weak base in 100.00 mL water and titrates the
s...
s...
Questions in other subjects:




Mathematics, 27.06.2019 16:20

Mathematics, 27.06.2019 16:20




English, 27.06.2019 16:20

 for weak base is
for weak base is 



![pOH=pK_a+\log(\frac{[salt]}{[base]})](/tpl/images/0555/4585/13872.png)
![pOH=pK_b+\log(\frac{[BNO_3]}{[BOH]})](/tpl/images/0555/4585/b566f.png)
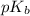 = negative logarithm of acid dissociation constant of formic acid = ?
= negative logarithm of acid dissociation constant of formic acid = ?
![[BNO_3]=\frac{0.004}{0.140}](/tpl/images/0555/4585/7cd20.png)
![[BOH]=\frac{0.006}{0.140} ](/tpl/images/0555/4585/f5b65.png)





