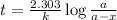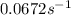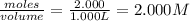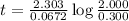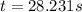At a certain fixed temperature, the reaction A(g) + 2 B(g) → AB2(g) is found to be first order in the concentration of A and zero order in the concentration of B. The reaction rate constant is 0.0672 s−1 . If 2.000 moles of A and 4.000 moles of B are placed in a 1.000 liter container, how many seconds will elapse before the concentration of A has fallen to 0.300 mol/liter? 1. 36.6948 2. 60.226 3. 63.2373 4. 34.244 5. 49.9242 6. 23.8631 7. 51.2735 8. 29.6425 9. 36.1356 10. 28.231 Answer in units of s.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 12:50, jholbrook7643
Which of these describes the rate of this chemical reaction? h2 + cl2 → 2 hcl a. an increase in the concentration of hcl and h2 with time b. an increase in the concentration of hcl with time c. an increase in h2 and cl2 with time d. a decrease in hcl and cl2 with time
Answers: 1
You know the right answer?
At a certain fixed temperature, the reaction A(g) + 2 B(g) → AB2(g) is found to be first order in th...
Questions in other subjects:








Mathematics, 16.10.2020 08:01


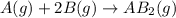
![Rate=k[A]^1[B]^0](/tpl/images/0555/2030/f8c47.png)