
Chemistry, 20.03.2020 01:34 sistrunkbrittnorn5dz
6.02 kJ>mol. When a small ice cube at -10°C is put into a cup of water at room temperature, which of the following plays a greater role in cooling the liquid water: the warming of the ice from -10°C to 0°C, or the melting of the ice?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

You know the right answer?
6.02 kJ>mol. When a small ice cube at -10°C is put into a cup of water at room temperature, which...
Questions in other subjects:



English, 01.07.2019 22:30







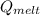 = latent heat of ice
= latent heat of ice

