
Chemistry, 20.03.2020 01:40 churchlady114p2met3
Write equations that show the processes that describe the first, second, and third ionization energies for a gaseous gadolinium atom. Express your answers as chemical equations separated by commas. Identify all of the phases in your answer.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Write equations that show the processes that describe the first, second, and third ionization energi...
Questions in other subjects:


Mathematics, 22.09.2019 02:30

History, 22.09.2019 02:30

Mathematics, 22.09.2019 02:30

Mathematics, 22.09.2019 02:30

Mathematics, 22.09.2019 02:30

Social Studies, 22.09.2019 02:30


Biology, 22.09.2019 02:30

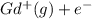 ,
, 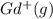 →
→ 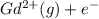 ,
, 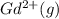 →
→ 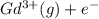
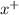 ion. The second ionization energy is always higher than the first:
ion. The second ionization energy is always higher than the first: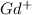 (g) →
(g) → 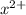 ion:
ion: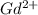 (g) →
(g) → 


