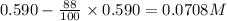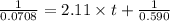Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M−1s−1NH32 Suppose a vessel contains NH3 at a concentration of 0.590M. Calculate how long it takes for the concentration of NH3 to decrease by 88.0%. You may assume no other reaction is important.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 04:00, josephicarusmarrujo
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1

Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 11:30, cjmckee2001
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

Chemistry, 23.06.2019 13:00, kayleegeise
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M...
Questions in other subjects:

Mathematics, 18.10.2020 05:01




English, 18.10.2020 05:01




Mathematics, 18.10.2020 05:01

World Languages, 18.10.2020 05:01

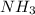 to decrease by 88.0%.
to decrease by 88.0%. 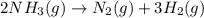
 , the kinetics must be second order.
, the kinetics must be second order.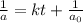
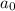 = initiaal concentration = 0.590 M
= initiaal concentration = 0.590 M