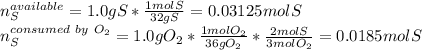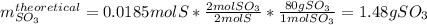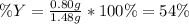Chemistry, 20.03.2020 00:28 madisonenglishp2qkow
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide, an environmental pollutant: 2S(s) + 3O2(g) → 2SO3(g) In a particular experiment, the reaction of 1.0 g S with 1.0 g O2 produced 0.80 g of SO3. The % yield in this experiment is .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide, an environmental pollu...
Questions in other subjects:

Mathematics, 09.05.2020 08:57

Chemistry, 09.05.2020 08:57


Mathematics, 09.05.2020 08:57

Biology, 09.05.2020 08:57

Mathematics, 09.05.2020 08:57


Biology, 09.05.2020 08:57