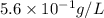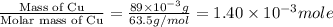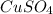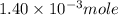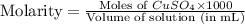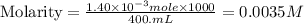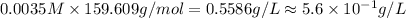Fe(s) + CuSO4(aq) <===> Cu(s) + FeSO4(aq)
Suppose an industrial quality-control ch...

Chemistry, 20.03.2020 00:39 xocupcake309174
Fe(s) + CuSO4(aq) <===> Cu(s) + FeSO4(aq)
Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 400.mL copper (II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 89.mg .
Calculate the original concentration of copper (II) sulfate in the sample. Round your answer to 2 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

You know the right answer?
Questions in other subjects: