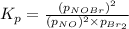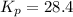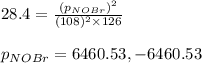Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1


Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Consider the reaction: 2 NO(g) + Br2(g) ∆ 2 NOBr(g) Kp = In a reaction mixture at equilibrium, the p...
Questions in other subjects:

English, 07.04.2021 01:40

Mathematics, 07.04.2021 01:40

Social Studies, 07.04.2021 01:40

Spanish, 07.04.2021 01:40




Mathematics, 07.04.2021 01:40

Mathematics, 07.04.2021 01:40

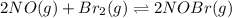
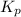 for above equation follows:
for above equation follows: