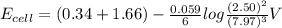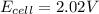Chemistry, 19.03.2020 23:58 naseersaad
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: →+3Cu+2aq2Als+3Cus2Al+3aq Suppose the cell is prepared with 7.97 M Cu+2 in one half-cell and 2.50 M Al+3 in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: →+3Cu+2aq2Als...
Questions in other subjects:

History, 04.02.2020 18:57

History, 04.02.2020 18:57



History, 04.02.2020 18:57


Biology, 04.02.2020 18:57


Biology, 04.02.2020 18:58

Chemistry, 04.02.2020 18:58

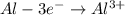 )
)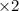 ;
; 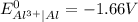
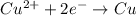 )
)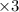 ;
; 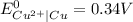
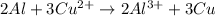
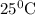 -
-![E_{cell}=[E_{Cu^{2+}\mid Cu}^{0}-E_{Al^{3+}\mid Al}^{0}]-\frac{0.059}{n}log\frac{[Al^{3+}]^{2}}{[Cu^{2+}]^{3}}](/tpl/images/0554/9233/c4d3d.png)