Chemistry, 20.03.2020 00:09 arieltaylor3924
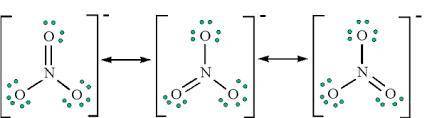
Why are the N-O bond lengths in NO− 3 the same? 1. The dot structure for this ion shows that all three bonds are single bonds. 2. The longer bond length is always used. 3. The shorter bond length is always used. 4. Resonance

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

You know the right answer?
Why are the N-O bond lengths in NO− 3 the same? 1. The dot structure for this ion shows that all thr...
Questions in other subjects:


Social Studies, 23.06.2019 18:30

Biology, 23.06.2019 18:30

Mathematics, 23.06.2019 18:30




Business, 23.06.2019 18:30


Mathematics, 23.06.2019 18:30