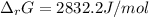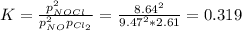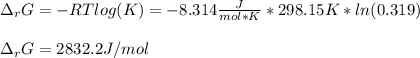Chemistry, 20.03.2020 00:06 nguyenhoangthienkim0
A chemist fills a reaction vessel with 9.47 atm nitrogen monoxide (NO) gas, 2.61 atm chlorine (C12) gas, and 8.64 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction:
2NO(g) + Cl2(g) <=> 2NOCI (g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
A chemist fills a reaction vessel with 9.47 atm nitrogen monoxide (NO) gas, 2.61 atm chlorine (C12)...
Questions in other subjects:


English, 11.02.2021 04:30

Social Studies, 11.02.2021 04:30

Arts, 11.02.2021 04:30

Mathematics, 11.02.2021 04:30

Mathematics, 11.02.2021 04:30

Mathematics, 11.02.2021 04:30