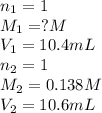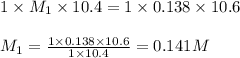Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 00:50, trinityine
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
An aqueous solution of hydroiodic acid is standardized by titration with a 0.138 M solution of potas...
Questions in other subjects:





Mathematics, 13.02.2020 22:38




English, 13.02.2020 22:38

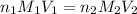
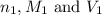 are the n-factor, molarity and volume of acid which is HI
are the n-factor, molarity and volume of acid which is HI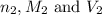 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.