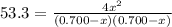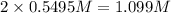Chemistry, 19.03.2020 23:27 shermoisllo3
At a certain temperature, the equilibrium constant, K c , Kc, for this reaction is 53.3. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 53.3 H2(g)+I2(g)↽−−⇀2HI(g)Kc=53.3 At this temperature, 0.700 mol H 2 0.700 mol H2 and 0.700 mol I 2 0.700 mol I2 were placed in a 1.00 L container to react. What concentration of HI HI is present at equilibrium?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Vicky22Shz
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
At a certain temperature, the equilibrium constant, K c , Kc, for this reaction is 53.3. H 2 ( g ) +...
Questions in other subjects:

Mathematics, 30.03.2021 21:40

Biology, 30.03.2021 21:40

Mathematics, 30.03.2021 21:40


Mathematics, 30.03.2021 21:40





![[H_2]=\frac{0.700 mol}{1.00 L}=0.700 M](/tpl/images/0554/8381/9fcad.png)
![[I_2]=\frac{0.700 mol}{1.00 L}=0.700 M](/tpl/images/0554/8381/1f00e.png)
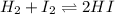
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0554/8381/62646.png)