
Chemistry, 19.03.2020 23:13 esmeralda266
Chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250.mL sample of groundwater known to be contaminated with iron(III) chloride, which would react with silver nitrate solution like this:FeCl3(aq) + 3AgNO3(aq) ⟶ 3AgCl(s) + FeNO3(aq)The chemist adds 82.0 M silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 2.5mg of silver chloride. Calculate the concentration of iron(III) chloride contaminant in the original groundwater sample.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, portedon8644
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Chloride anions in solution will combine with the silver cations to produce bright white silver chlo...
Questions in other subjects:





Mathematics, 15.02.2022 01:40



English, 15.02.2022 01:40

Mathematics, 15.02.2022 01:40

Chemistry, 15.02.2022 01:40

 the concentration of iron(III) chloride contaminant in the original groundwater sample.
the concentration of iron(III) chloride contaminant in the original groundwater sample.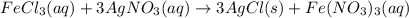
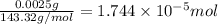
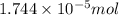 od silver chloride will be obtained from ;
od silver chloride will be obtained from ;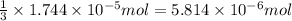 of ferric chloride
of ferric chloride![[FeCl_3]=\frac{5.814\times 10^{-6} mol}{0.250 L}=2.326\times 10^{-5} M](/tpl/images/0554/8189/91194.png)


