
Chemistry, 19.03.2020 22:45 anthonylopez1
When aluminum, AlAl, metal is dipped in an aqueous solution of hydrochloric acid, HClHCl, hydrogen gas, H2H2, is produced with the formation of an aluminum chloride, AlCl3AlCl3, solution. Write the balanced chemical equation showing the phases of reactants and products.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3
You know the right answer?
When aluminum, AlAl, metal is dipped in an aqueous solution of hydrochloric acid, HClHCl, hydrogen g...
Questions in other subjects:










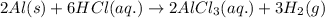
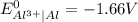 ).
).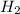 is present at the intermediate position in electrochemical series with a standard reduction potential of 0 V (
is present at the intermediate position in electrochemical series with a standard reduction potential of 0 V (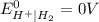 ).So, when Al is dipped in aqueous solution of HCl, Al is readily oxidized to produce
).So, when Al is dipped in aqueous solution of HCl, Al is readily oxidized to produce 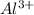 whereas
whereas 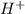 is reduced to
is reduced to 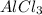 and gaseous
and gaseous 

