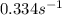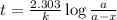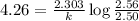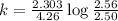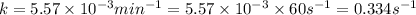Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
The decomposition of N_2O_5(g) following 1st order kinetics. N_2O_5(g) to N_2O_4(g) + ½ O_2(g) If 2....
Questions in other subjects:


English, 30.04.2021 16:30


Mathematics, 30.04.2021 16:30

Mathematics, 30.04.2021 16:30





Mathematics, 30.04.2021 16:30