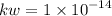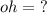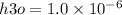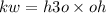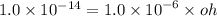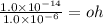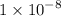1.0 x 10-6 M


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
What is the [OH-] in a solution that has a [H3O+] = 1.0 x 10-6 M?
1.0 x 10-6 M
1.0 x 10-6 M
Questions in other subjects:


Mathematics, 19.05.2021 21:00




Mathematics, 19.05.2021 21:00

Mathematics, 19.05.2021 21:00

History, 19.05.2021 21:00