
Chemistry, 19.03.2020 21:01 gokusupersaiyan12345
According to the Bronsted-Lowry definition,
an acid is a proton acceptor
a base is a proton donor
a base produces H+ ions in aqueous solutions
a base is a proton acceptor

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, 20heldmadison
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

You know the right answer?
According to the Bronsted-Lowry definition,
an acid is a proton acceptor
a b...
an acid is a proton acceptor
a b...
Questions in other subjects:





Geography, 30.06.2019 03:50





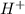 + Conjugate base
+ Conjugate base


